June 5, 2017
Denosumab delays the time to first skeletal-related event in patients with myeloma
Denosumab delays the time to first skeletal-related event in patients with myeloma
53rd Annual Meeting of the American Society of Clinical Oncology (ASCO)
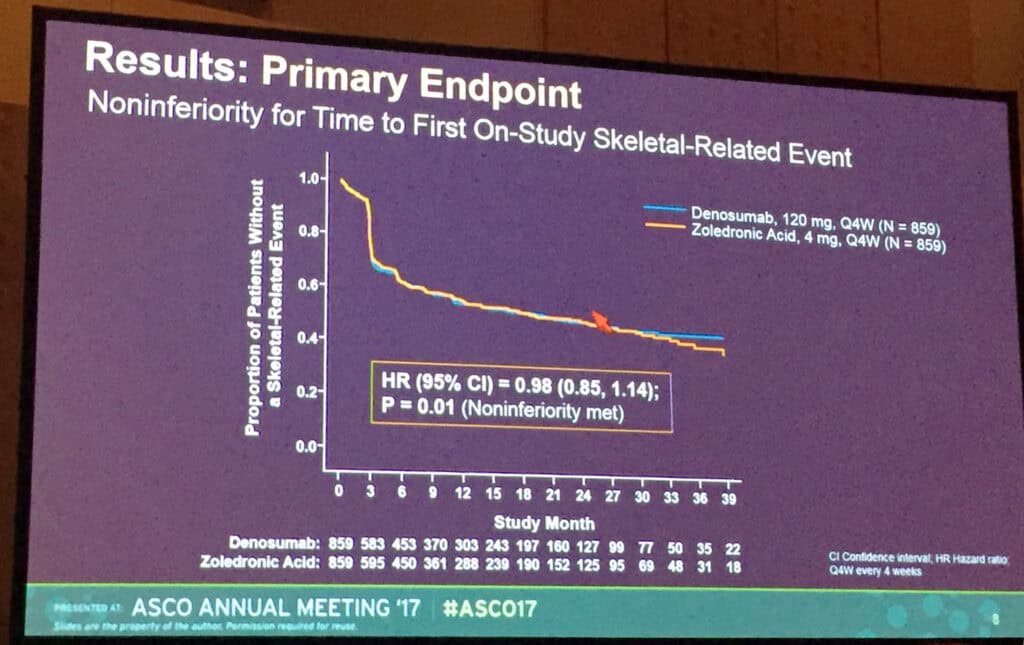
Chicago – According to Phase 3 ‘482 study, the largest international multiple myeloma trial ever conducted with 1,718 patients involved, patients who receive denosumab (XGEVA) had a significantly lower rate of renal adverse events compared to zoledronic acid (10.0 percent versus 17.1 percent, p<0.001). The study met its primary endpoint, demonstrating denosumab is non-inferior to zoledronic acid in delaying the time to first on-study skeletal-related event in patients with myeloma (HR=0.98, 95 percent CI: 0.85, 1.14; p=0.01). ). These results was presented yesterday at the 53rd Annual Meeting of the American Society of Clinical Oncology (ASCO).
The results presented showed that in patients with renal insufficiency, defined as having a creatinine clearance (CrCl) of less than or equal to 60 mL/min, the rate of renal adverse events was double in the zoledronic acid arm compared to the denosumab arm (26.4 percent versus 12.9 percent, respectively). The zoledronic acid label does not recommend treatment in patients with a CrCl of less than 30 mL/min; therefore, patients with severe renal impairment were excluded from this study.1 Median cumulative exposure to denosumab was 15.75 months compared to 14.78 months for zoledronic acid.
“Bone complications in myeloma patients, including fractures, can have a devastating impact on patients. Current treatment options for these complications are limited to bisphosphonates, including zoledronic acid, which are cleared by the kidneys and can be associated with increased renal toxicity,” said Noopur Raje, M.D., director, Center for Multiple Myeloma, Massachusetts General Hospital Cancer Center, Boston. “Renal impairment is a common complication in multiple myeloma patients. Denosumab, which is not cleared by the kidneys, may offer a novel, safe and effective option for multiple myeloma patients.”
Denosumab is currently indicated for the prevention of fractures and other skeletal-related events in patients with bone metastases from solid tumors. This drug is currently not indicated for the prevention of skeletal-related events in myeloma patients. On April 4, 2017, Amgen announced the submission of a supplemental Biologics License Application to the U.S. Food and Drug Administration (FDA) and an application for a variation to the marketing authorization to the European Medicines Agency (EMA) for this drug. The submissions to regulatory authorities seek to expand the currently approved denosumab indication to include patients with myeloma.
Myeloma and bone complications
Myeloma is the second most common hematologic cancer, and it develops in plasma cells located in the bone marrow microenvironment. It is typically characterized by osteolytic bone lesions as well as renal failure, which are both part of diagnosis (CRAB criteria). Each year an estimated 114,000 new cases of myeloma are diagnosed worldwide, resulting in more than 80,000 deaths per year.
More than 90 percent of patients develop osteolytic lesions during the course of the disease. Preventing bone complications is a critical aspect of caring for patients with this disease, because these events can cause significant morbidity. Current treatment options for fractures and other bone complications are limited to bisphosphonates, including zoledronic acid, which are cleared through the kidneys. Approximately 60 percent of all myeloma patients have or will develop renal impairment over the course of the disease.