The SARS-CoV-2 (COVID-19) pandemic has raised many questions, especially around vaccination. We hope to answer some of the most frequently asked questions about the current approved vaccines and treatments in Europe. This is not intended to be medical advice; as every individual case differs, we recommend you consult your haematologist or a clinician, if you have further questions or concerns. As the situation surrounding COVID-19 and vaccination is evolving rapidly, MPE will attempt to update this document when new information emerges (last update 2/2022). This Q&A provides information on the following:
- The vaccines approved by the European Medicines Agency (EMA) as of February 2022
- Vaccines under review by the EMA as of February 2022
- How COVID-19 vaccines work and are administered
- The efficacy of the COVID-19 vaccines in the general population and myeloma patients
- Risks and recommendations around COVID-19 vaccination
- The need for a vaccine booster
- Approved treatments for COVID-19 in Europe as of February 2022
- Treatments under review, or submitted for authorisation in Europe, as of February 2022
- The impacts of COVID-19 on the myeloma patient population
Disclaimer:
This is not intended to be medical advice. Please speak with your clinician, if you have any questions or concerns about COVID-19 vaccination or treatment.
Approved COVID-19 Vaccines in Europe
Which COVID-19 vaccines are approved by the European Medicines Agency (EMA)?
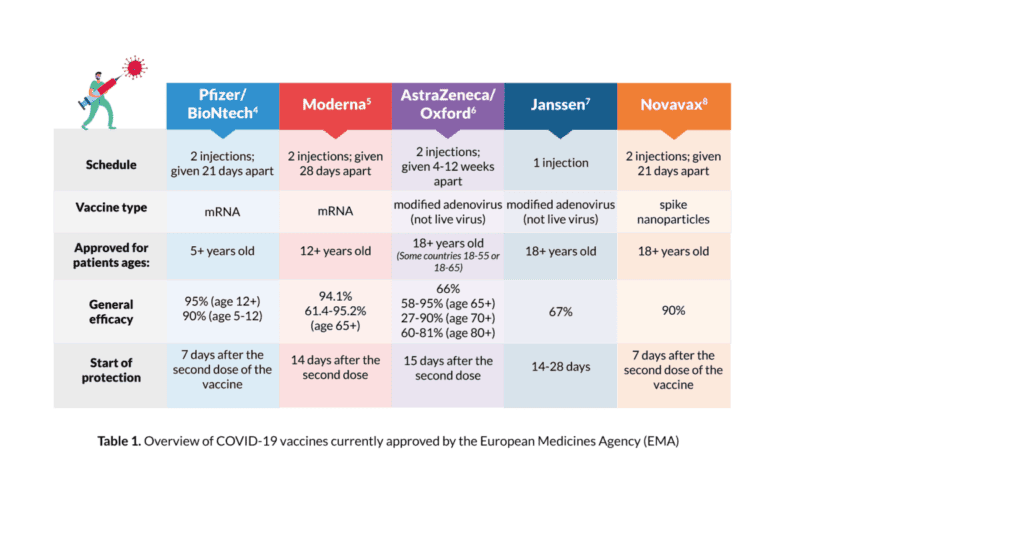
There are currently five vaccines approved for use by the EMA for the prevention of COVID-19 (see Table 1 for more information on each vaccine and its efficacy in the general population):
- BioNTech/Pfizer COVID-19 vaccine (Comirnaty); for individuals age five and older
- AstraZeneca/Oxford COVID-19 vaccine (Vaxzevria); for individuals age 18 and older (some countries 18-55 or 18-65)
- Moderna COVID-19 vaccine (Spikevax); for individuals age 12 and older
- Janssen COVID-19 vaccine; for individuals age 18 and older
- Novavax (Nuvaxovid); for individuals age 18 and older
Find the latest and up-to-date information on approved COVID-19 vaccines in your country here.
What type of approval process did these vaccines undergo?
Due to the existing and continued public health emergency, COVID-19 vaccines went through an accelerated development process but were still subject to the rigorous evaluation methods used by the EMA. These vaccines were evaluated against the same high standards as any other medicine. It is essential to understand that while approval for the COVID-19 vaccines was accelerated, vaccines with similar mechanisms of action to the EMA-approved COVID-19 vaccines have been in development for years. Therefore, the current COVID-19 vaccines were developed using existing research.
How do the approved COVID-19 vaccines work?
Vaccines teach our immune system to recognise and protect us from COVID-19 if/ when we get infected with the virus. The BioNTech/Pfizer and Moderna vaccines are mRNA vaccines. These vaccines use genetic material called mRNA, which, after injection, enters your cells (note: this genetic material does not enter the innermost part of your cells called the nucleus nor your DNA). The cells translate the mRNA into proteins that look like the proteins found on the surface of the COVID-19 virus. These proteins then enter your bloodstream, where the body recognises them as foreign and generates an immune response. This means, if you contract COVID-19 after receiving the vaccination, your body will recognise the virus and be able to respond quickly to fight the infection.
The AstraZeneca/Oxford and Janssen vaccines use a different approach with viral vectors. These vaccines are made using an inactive adenovirus, which serves as a shell to carry DNA genetic material into your cells (note: this genetic material does not enter the innermost part of your cells called the nucleus nor your DNA). This DNA is then made into mRNA and then into proteins that look like the proteins found on the surface of the COVID-19 virus. These proteins then enter your bloodstream, where the body recognises them as foreign and generates an immune response. Therefore, if you contract COVID-19 after receiving the vaccination, your body will recognise the virus and be able to respond quickly to fight the infection. The Novavax vaccine injects proteins called “spike nanoparticles” into the body. Researchers selected the genes that encode spike proteins and inserted them into a baculovirus, an insect virus. The virus infects moth cells, enforcing them to produce the spike protein, which are then collected by researchers and fused with nanoparticles. These nanoparticles resemble the proteins found on the surface of COVID-19 and are recognised by the body as foreign to stimulate an immune response. Therefore, if you contract COVID-19 after receiving the vaccination, your body will recognise the virus and be able to respond quickly to fight the infection. None of these vaccines contain live viruses, and there is no risk of catching COVID-19 (or adenovirus/baculovirus) from the vaccine.
How are the approved COVID-19 vaccines administered?
For initial vaccination, the BioNTech/Pfizer, Moderna, Novavax, and AstraZeneca/ Oxford vaccines are given via injection in two doses at specific intervals (see Table 1 for more information). A third vaccination or booster is recommended after the first two doses. See the booster section for more information. For initial vaccination, the Janssen vaccine is given via injection in one dose (see Table 1 for more information). A second vaccination or booster is recommended after the first dose. See the booster section for more information. All the injections are usually given into the muscle of the upper arm.
How effective are the approved vaccines?
While each vaccine has different efficacy, they all offer protection against hospitalisation and death from COVID-19. Also, given the timing, population and location of where each vaccine was studied, it is impossible to compare their efficacy, and judge any single vaccine to be either inferior or superior to any other. While the efficacy of these vaccines in the face of emerging variants is unknown, it is still important to note that getting vaccinated remains the best way to provide yourself with some level of protection against COVID-19. Vaccines with lower effectiveness can still save thousands of lives and prevent millions of cases of COVID-19. The flu shot, for example, has an effectiveness of 40–60%, according to the United States Centers for Disease Control and Prevention (CDC). However, during 2018–2019, it prevented around “4.4 million influenza illnesses, 2.3 million influenza-associated medical visits, 58,000 influenza- associated hospitalisations and 3,500 influenza-associated deaths in the US.”1,2 Each of the COVID-19 vaccines was tested in thousands of patients of varying ethnicities and some with medical conditions, for example, lung and heart disease, but were otherwise initially mostly tested in individuals without cancer. Further known and emerging data on the efficacy in myeloma patients is detailed below. See Table 1 for a summary of efficacy information for each approved vaccine. Of note, although the EMA has approved the AstraZeneca/Oxford vaccine for patients aged 18 and older, some countries restrict this to patients aged 18 to 55 or aged 18 to 65 years as there is some concern that this vaccine may be less effective in older patients, or have a higher risk of side effects in individuals of a certain age.
How effective are the approved vaccines in myeloma patients?
Several studies have been published investigating antibody levels after vaccination in the myeloma population. These studies often include small sample sizes and are not designed in ways where the data is generalisable to the greater myeloma population. Given the effects of myeloma and its treatment, and the immunosuppressed state of myeloma patients, it is likely that the COVID-19 vaccines may not be as effective in myeloma patients as they are in the general population. Therefore, getting the COVID-19 vaccine may not ensure full immunity and you must still physically distance, wear a mask and wash your hands. In a pooled analysis of 1,564 myeloma patients, the overall antibody response after full COVID-19 vaccination (i.e. one or two doses depending on the vaccine type) was found to be about 76% (as compared to about 100% in the general population).3 See Appendix I for data on individual studies of the efficacy in myeloma patients.
How soon after receiving a vaccine am I considered to be protected? How long does protection last?
After vaccination, you are not considered protected until sometime after the second dose. Therefore, it is important to continue to limit your risk of catching or transmitting COVID-19 before you get your second shot. Ways to limit your risk of catching or transmitting COVID-19 are to physically distance two metres from others when out in public, wash your hands or use sanitiser frequently, avoid touching your face, avoid large crowds and wear a mask. Research has shown that around six months after your second dose of vaccine you may require a booster shot as the immunity from the initial two doses decreases over time. See the booster section for more information on this topic. See Table 1 for specifics on the timing of protection for each approved vaccine.
Do the approved COVID-19 vaccines protect against variants?
As a virus spreads it can adapt and mutate (change its genetic sequencing) to potentially become more or less transmissible and infectious. The new (mutated) COVID-19 virus is then called a “variant.” The World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDPC) have identified several “variants of concern” which are rapidly spreading worldwide.9 According to the EMA, the scientific community and regulators are closely monitoring how SARS-CoV-2 (the virus that causes COVID-19) changes over time, and how well COVID-19 vaccines can protect people against COVID-19 caused by any new variants of the virus that appear. The EMA has asked all COVID-19 vaccine developers to investigate whether their vaccine can offer protection against any new variants and to submit relevant data. There is some preliminary information on how these vaccines work against variants of concern. A summary of this information can be found in Table 2.
What are the side effects of the COVID-19 vaccines?
Studies investigating the COVID-19 vaccine were done on many patients and showed there is a very low risk of serious side effects from all five vaccines. The most common side effects were chills, headache, pain (generalised body pain or at the injection site), fever, nausea, tiredness and/or redness, and swelling at the injection site. Most of the side effects were considered mild or moderate and resolved within a day or two after the vaccination. On rare occasions, individuals developed severe allergic reactions (anaphylaxis), inflammation of the heart (myocarditis), blood clots, or low platelets (also known as vaccine-induced immune thrombotic thrombocytopenia) soon after vaccination. In most cases, the benefits of the vaccine outweigh these risks and COVID-19 vaccines should be given under close medical supervision to monitor potential side effects. Anyone with a history of severe allergic reactions or adverse events can potentially still receive these vaccines, but they should first consult their doctor to discuss the risks and benefits prior to vaccination.
Are there any recommendations surrounding COVID-19 vaccination for myeloma patients?
According to a consensus of European myeloma experts (the European Myeloma Network [EMN]):11
- Patients should be vaccinated during times when their myeloma is well controlled or when they are off treatment, before the start of therapy or stem cell collection, or three months after stem cell This timeline may be modified depending on the circumstances of the pandemic.
- While the routine evaluation of immune response after vaccination is not recommended, according to the EMN, it may allow for the identification of patients with no or low vaccine response. Therefore after evaluation of antibody levels, the EMN recommends the following as it pertains to patients that have not responded:
- These patients should receive an additional dose of vaccine
- These patients should continue to physically distance and reduce their risk of infection
- These patients should ensure that their carers and close contacts are all vaccinated
- These patients may require monoclonal antibodies if they come into contact with or have been exposed to someone with COVID-19
Given your immunocompromised state, it is important that those around you, especially your carers, receive their COVID-19 vaccine. When those around you are considered to be immune to COVID-19 this lowers the overall amount of virus spread and lowers the risk that you will become infected if your carer happens to catch COVID-19. This concept is known as “herd immunity.”12
Do I need a booster shot, why, and when?
As the COVID-19 virus spreads the world has seen an emergence of variants with higher levels of infectivity. Research has shown that some vaccinations, after two doses, do not provide enough protection for myeloma patients or against the new emerging variants. According to the EMA, it is important to distinguish between a third dose of vaccine (given specifically to individuals weakened immune systems) and a booster dose of vaccine.13 A third dose of a vaccine is given to people who are considered immunocompromised (often about 28 days after their last vaccination) and may not develop adequate immunity after full vaccination. According to the World Health Organization, a third dose is considered essential to complete the primary series of vaccination for better protection against COVID-19.14 A booster dose of a vaccine can be the original or a lower dose of vaccine given to individuals after their primary vaccination is complete (often about six months later). A booster dose is used to improve immunity as it decreases over time after primary vaccination.14 A third dose of the Moderna and Pfizer/BioNTech vaccines was investigated in individuals that had undergone organ transplantation and were considered severely immunocompromised. This research found that the administration of a third dose of vaccine 28 days after their second dose resulted in a significant increase in antibody levels thought to have a clinical impact on the protection of individuals that are immunocompromised. This study supported the approval of a third dose of vaccine for immunocompromised patients.15
Figure 2. Recommended vaccination schedule for approved vaccines in Europe. As more data is collected, it is likely that additional vaccine doses may be recommended for those with severe immunosuppression and insufficient responses to previous doses. Furthermore, research that evaluated a booster dose six months after receiving a second dose of vaccine showed improvement in antibody levels in the general population. As a result, the EMA has now approved all available vaccines for a booster dose to improve protection from COVID-19.13,16 See Figure 2 for the recommended vaccine schedule for myeloma/immunocompromised patients.
How does the COVID-19 vaccine affect treatment for myeloma and AL amyloidosis?
There is no evidence that the vaccine will have any interactions with the medicines used to treat myeloma and AL amyloidosis. However, some drugs (such as daratumumab, stem cell transplant, or anti-BCMA treatment) used during intensive treatment may weaken the immune system and therefore weaken the body’s response to the COVID-19 vaccine. As a result, patients undergoing intensive therapy may not respond as well to the COVID-19 vaccine and some clinicians may recommend vaccination at specific times in a treatment cycle. Despite this, it is still recommended to get the vaccine. Patients should consult their doctor/haematologist to determine their options and appropriate timing of vaccination based on their individualised treatment regimen and situation.
What are the risks of choosing not to get vaccinated?
While research has shown that immune response to vaccination in myeloma patients may be impaired, data shows that vaccines can be effective and beneficial in myeloma patients to provide protection from hospitalisation and/or death from COVID-19. Choosing not to get vaccinated may result in death from COVID-19 or risk for irreversible damage to your lungs or heart with a severe impact on your quality of life. Multiple myeloma patients are at a twofold higher risk to become infected by the SARS-CoV2 virus. The risk of death from COVID-19 has been estimated to be as high as 54% in myeloma patients that contract COVID-19.30 Other research has shown that patients that have recovered from COVID-19 may develop chronic symptoms such as fatigue, shortness of breath, difficulty thinking (“brain fog”), cough, chest/stomach pain, racing heart, body pains or aches, diarrhoea, mood changes, loss of smell or taste, rash, fever and organ problems/damage (heart, lung, kidney, skin, and brain).31
COVID-19 Vaccinations Under Review in Europe
Which COVID-19 vaccines are under review by the European Medicines Agency (EMA)?
There are currently four vaccines under rolling review by the EMA for the prevention of COVID-19 as of February 2022 (see Table 3 for more information on each vaccine):
- Sputnik V Gam-COVID-Vac (Gamaleya Institute)
- COVID-19 Vaccine (Vero Cell) Inactivated (Sinovac)
- Vidprevtyn (Sanofi)
- VLA2001 (Valneva)
Treatment for COVID-19 in Europe
Which COVID-19 treatments are approved by the European Medicines Agency (EMA)?
There are currently seven COVID-19 treatments approved for use by the European Medicines Agency for the treatment of COVID-19 (see Table 4a and 4b for more information on each treatment):
- Kineret (anakinra)
- Paxlovid (ritonavir)
- Regkirona (regdanvimab)
- RoActemra (tocilizumab)
- Ronapreve (casirivimab / imdevimab)
- Veklury (remdesivir)
- Xevudy (sotrovimab)
Promising treatments for COVID-19 under investigation and not yet available in Europe
There are several other treatments that have shown promising efficacy and are available in other countries such as the United States. These treatments are monoclonal antibodies that work similarly to the already approved treatments. Monoclonal antibodies are being considered as an option for myeloma patients for preventative/routine treatment to prevent COVID-19 infection, especially in those that do not respond to vaccination. However, this is not yet approved or widely used in Europe at this time. It should be noted that the efficacy of these treatments varies, depending on the current circulating variants. Previous studies have shown that one dose of a monoclonal antibody (bamlanivimab) reduced the rate of new COVID-19 infections in the staff and residents at a nursing home by 57% and mortality by 100%.11 The drug combination bamlanivimab/etesevimab, likewise, is another effective monoclonal antibody combination available in the United States for individuals who have been exposed to COVID-19, or who are at risk of developing severe infection.11 This combination of antibodies, however, does not appear to be effective against the current circulating variant, Omicron.
Which COVID-19 treatments have been submitted or are being reviewed for market authorisation by the European Medicines Agency (EMA)?
There is currently one COVID-19 treatment under rolling review by the European Medicines Agency for the treatment of COVID-19:29
- Evusheld (tixagevimab/cilgavimab) is being developed by AstraZeneca and is a combination of monoclonal antibodies (laboratory engineered antibodies) that recognise the surface (spike) protein of COVID-19 to support the immune system response to COVID-19 infections and prevent the virus from entering the cells. Evusheld, however, does not appear to be effective against the Omicron variant.
There are currently two COVID-19 treatments that have marketing authorisation applications submitted to the European Medicines Agency for approval for the treatment of COVID-19:29
- Lagevrio (molnupiravir) is a medication under development by Merck Pharmaceuticals and is a drug that prevents the replication of the COVID-19 virus inside the body.
- Olumiant (baricitinib) is a medication under development by Eli Lilly that is already authorised in the EU for the treatment of rheumatoid This drug blocks specific enzymes that cause inflammation and tissue damage seen in patients who are suffering from severe COVID-19 infection.
References
- Estimated Influenza Illnesses, Medical Visits, and Hospitalizations Averted by Vaccination | Published August 26, 2021. Accessed January 1, 2022. https://www.cdc.gov/flu/vaccines-work/burden-averted.htm
- Vaccine Effectiveness: How Well Do Flu Vaccines Work? | CDC. Published October 25, Accessed January 1, 2022. https://www.cdc.gov/flu/ vaccines-work/vaccineeffect.htm
- Gagelmann N, Passamonti F, Wolschke C, et Antibody response after vaccination against SARS-CoV-2 in adults with haematological malignancies: a systematic review and meta-analysis. Haematologica. Published online December 16, 2021. doi:10.3324/haematol.2021.280163
- Comirnaty | European Medicines Accessed January 14, 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty
- CZARSKA-THORLEY Spikevax (previously COVID-19 Vaccine Moderna). European Medicines Agency. Published January 4, 2021. Accessed January 14, 2022. https://www.ema.europa.eu/en/medicines/ human/EPAR/spikevax
- DIMITROVA EK. Vaxzevria (previously COVID-19 Vaccine AstraZeneca). European Medicines Published January 25, 2021. Accessed January 14, 2022. https://www.ema.europa.eu/en/medicines/human/ EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca
- FRANCISCO EM. COVID-19 Vaccine Janssen. European Medicines Agency. Published March 5, 2021. Accessed January 14, 2022. https://www.ema. eu/en/medicines/human/EPAR/covid-19-vaccine-janssen
- FRANCISCO Nuvaxovid. European Medicines Agency. Published December 17, 2021. Accessed January 14, 2022. https://www.ema. europa.eu/en/medicines/human/EPAR/nuvaxovid
- SARS-CoV-2 variants of concern as of 13 January 2022. Accessed January 14, https://www.ecdc.europa.eu/en/covid-19/variants-concern
- Ramesh S, Govindarajulu M, Parise RS, et Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy. Vaccines. 2021;9(10):1195. doi:10.3390/vaccines9101195
- Ludwig H, Sonneveld P, Facon T, et COVID-19 vaccination in patients with multiple myeloma: a consensus of the European Myeloma Network. Lancet Haematol. 2021;8(12):e934-e946. doi:10.1016/S2352-3026(21)00278-7
- Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19. Accessed January 16, 2022. https://who.int/news-room/ questions-and-answers/item/herd-immunity-lockdowns-and-covid-19
- HRABOVSZKI Comirnaty and Spikevax: EMA recommendations on extra doses boosters. European Medicines Agency. Published October 4, 2021. Accessed January 14, 2022. https://www.ema.europa.eu/en/news/ comirnaty-spikevax-ema-recommendations-extra-doses-boosters
- Coronavirus disease (COVID-19): Accessed January 16, 2022. https://www.who.int/news-room/questions-and-answers/item/ coronavirus-disease-(covid-19)-vaccines
- Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a Third Dose of mRNA- 1273 Vaccine in Transplant Recipients. N Engl J Med. 2021;385(13):1244- doi:10.1056/NEJMc2111462
- Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS- CoV-2 omicron 1.1.529 variant by post-immunisation serum. Lancet Lond Engl. Published online December 20, 2021:S0140-6736(21)02844- 0. doi:10.1016/S0140-6736(21)02844-0
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626-636. doi:10.1038/s41577-021-00592-1
- EMA starts rolling review of the Sputnik V COVID-19 vaccine | European Medicines Agency. Accessed January 14, 2022. https://ema.europa. eu/en/news/ema-starts-rolling-review-sputnik-v-covid-19-vaccine
- EMA starts rolling review of COVID-19 Vaccine (Vero Cell) Inactivated | European Medicines Accessed January 14, 2022. https://www. ema.europa.eu/en/news/ema-starts-rolling-review-covid-19-vaccine- vero-cell-inactivated
- PINHO EMA starts rolling review of COVID-19 vaccine Vidprevtyn. European Medicines Agency. Published July 20, 2021. Accessed January 14, 2022. https://www.ema.europa.eu/en/news/ema-starts-rolling- review-covid-19-vaccine-vidprevtyn
- BERBECE EMA starts rolling review of Valneva’s COVID-19 vaccine (VLA2001). European Medicines Agency. Published December 2, 2021. Accessed January 14, 2022. https://www.ema.europa.eu/en/news/ema- starts-rolling-review-valnevas-covid-19-vaccine-vla2001
- European Medicines Agency. Published September 17, 2018. Accessed January 14, 2022. https://www.ema.europa.eu/en/medicines/ human/EPAR/kineret
- European Medicines Agency. Published January 24, 2022. Accessed February 3, 2022. https://www.ema.europa.eu/en/medicines/ human/EPAR/paxlovid
- FRANCISCO Regkirona. European Medicines Agency. Published November 10, 2021. Accessed January 14, 2022. https://www.ema. europa.eu/en/medicines/human/EPAR/regkirona
- European Medicines Agency. Published September 17, 2018. Accessed January 14, 2022. https://www.ema.europa.eu/en/medicines/ human/EPAR/roactemra
- FRANCISCO Ronapreve. European Medicines Agency. Published November 10, 2021. Accessed January 14, 2022. https://www.ema. europa.eu/en/medicines/human/EPAR/ronapreve
- DIMITROVA Veklury. European Medicines Agency. Published June 23, 2020. Accessed January 14, 2022. https://www.ema.europa.eu/en/ medicines/human/EPAR/veklury
- BUCKINGHAM Xevudy. European Medicines Agency. Published December 15, 2021. Accessed January 14, 2022. https://www.ema. europa.eu/en/medicines/human/EPAR/xevudy
- PINHO COVID-19 treatments. European Medicines Agency. Published February 18, 2021. Accessed January 14, 2022. https://www.ema.europa. eu/en/human-regulatory/overview/public-health-threats/coronavirus- disease-covid-19/treatments-vaccines/covid-19-treatments
- Matta S, Chopra KK, Arora Morbidity and mortality trends of Covid 19 in top 10 countries. Indian J Tuberc. 2020;67(4S):S167-S172. doi:10.1016/j. ijtb.2020.09.031
- The Centers for Disease Control and Post COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index. html
- Lockmer S, Uttervall K, Kashif M, et al. Antibody response to COVID-19 mRNA vaccine (Comirnaty) in myeloma patients treated with high-dose melphalan and/or immunotherapy. Am J Hematol. 2021;96(11):E443-E446. doi:10.1002/ajh.26348
- Shah MR, Gabel A, Beers S, Salaru G, Lin Y, Cooper DL. COVID-19 Vaccine Responses in Patients With Plasma Cell Dyscrasias After Complete Clin Lymphoma Myeloma Leuk. Published online November 11, 2021. doi:10.1016/j.clml.2021.11.001
- Bitoun S, Henry J, Vauloup-Fellous C, et al. Response to COVID-19 mRNA vaccination in multiple myeloma is conserved but impaired compared to J Hematol OncolJ Hematol Oncol. 2021;14(1):166. doi:10.1186/ s13045-021-01183-2
- Stampfer SD, Goldwater MS, Jew S, et Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021;35(12):3534-3541. doi:10.1038/s41375-021-01354-7
- Rahav G, Lustig Y, Lavee J, et BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine. 2021;41:101158. doi:10.1016/j.eclinm.2021.101158
- Ghandili S, Schönlein M, Lütgehetmann M, et Post-Vaccination Anti- SARS-CoV-2-Antibody Response in Patients with Multiple Myeloma Correlates with Low CD19+ B-Lymphocyte Count and Anti-CD38 Treatment. Cancers. 2021;13(15):3800. doi:10.3390/cancers13153800
Appendix I: COVID-19 Vaccination Efficacy in the Myeloma Population
In a study conducted in Greece,11 276 patients with active or smouldering myeloma or MGUS were enrolled in a clinical trial to measure the efficacy of the AstraZeneca and Pfizer/BioNTech vaccines as compared to healthy patients of the same age, receiving vaccination at the same time. In general, myeloma patients had lower neutralising (antibodies with the ability to destroy COVID-19) antibody levels (57%) compared to health controls (81%) 50 days after vaccination with the Pfizer/BioNTech vaccine or seven weeks after the first dose of the AstraZeneca vaccine. Furthermore, in a UK study,11 COVID-19 antibodies were measured in 93 patients after one dose of either the Pfizer/BioNTech or AstraZeneca vaccines. After 33 days, 52% of patients were found to have COVID-19 antibodies with no significant difference between the vaccines. 82% of patients (9/11) vaccinated within 12 months of stem cell transplant tested positive for COVID-19 antibodies. In a study conducted in Sweden,32 the Pfizer/BioNTech vaccine was tested in 93 patients (median age 62.4 years) that received high-dose melphalan (a chemotherapy agent) and/or immunotherapy. The participants spike protein levels (protein on the surface of COVID-19) were then tested four weeks after their second dose of vaccine. Patients on therapy (62 patients) received vaccine doses 3-4 days apart from administration of antimyeloma treatments and at least three months after stem cell transplant. 73% of the patients in this study developed antibodies after vaccination. Patients that were considered on treatment showed lower levels of spike proteins. It was found that patients that were treated with anti-BCMA treatment, daratumumab (more than once a month), or dexamethasone showed even lower levels of spike protein. When these patients were removed from data calculations there was no effect of other treatments on spike protein levels. Therefore, according to the authors, “this suggests that in general, disease-related, ongoing treatment besides treatment with anti-BCMA, daratumumab (more than once per month) and dexamethasone did not have an impact on spike protein levels.” The investigators also found that relapsed/refractory myeloma patients had lower spike protein levels than newly diagnosed myeloma patients. Notably, the study did not find high dose treatment before stem cell transplant to have any effect on spike protein levels. A real-world study,33 investigated the efficacy of the Moderna, Pfizer/BioNTech, and the Janssen vaccines in 122 patients with active or smouldering myeloma, a solitary plasmacytoma, and AL amyloidosis (collectively “plasma cell dyscrasias”). Overall, 95% of participants responded to vaccination. There was high response after the Pfizer/BioNTech and Moderna vaccines compared to the Janssen vaccine. But patients undergoing treatment with daratumumab, who were older, and who had received the Janssen vaccine had significantly lower levels of antibody response. The target antibody level (according to animal studies) after vaccination was thought to be 100 IU/mL in this study and after vaccination:
- 27% of participants (3/11) who received the Janssen vaccine had reached target antibody levels or higher
- 61% of participants (25/41) who received the BioNTech/Pfizer vaccine had reached target antibody levels or higher
- 76% of participants (28/37) who received the Moderna vaccine had reached target antibody levels or higher
In another study conducted in France,34 researchers compared the efficacy of the Pfizer/BioNTech vaccine in 37 myeloma patients versus 28 healthy individuals. Antibody levels were measured one month after the second dose of vaccine and found that while myeloma patients showed lower antibody levels, overall, 89% of myeloma patients (versus 97% of healthy individuals) developed an immune response. This study also found that 75% of myeloma patients achieved neutralising (antibody’s ability to destroy COVID-19) levels of antibodies (>50 IU/ mL) versus 96% in health controls. In a study conducted in the United States,35 the Pfizer/BioNTech vaccine was investigated in 103 myeloma patients (most with active disease). Blood antibody levels were tested between 12-21 days prior to first vaccination and 14-21 days following the first and second vaccinations. Antibody levels were found to be:
- Clinically relevant: greater than 250 IU/mL
- Partial responders: between 50-250 IU/mL
- Non responders: less than 50 IU/mL
The researchers found that individuals with smouldering myeloma responded better to vaccines and only 45% of patients with active myeloma developed a clinically relevant response. 22% of participants had a partial response to their vaccine. Patients with lower antibody levels were older, had kidney disease, lower immune cell (lymphocyte) counts, were on their second line (or greater) treatment and had not achieved complete remission. The study also found that participants had higher levels of response to the Moderna vaccine than Pfizer/BioNTech. In a study conducted in Italy,11 researchers found that myeloma patients that had received the Pfizer/BioNTech vaccine were found to have antibody levels of 7.5 AU/mL three weeks after their first dose of vaccine (compared to 12.1 AU/ mL in the general population) and 106.7 AU/mL (compared to 353.5 AU/mL in the general population) two weeks after their second dose of vaccine. In this research, vaccine “responders” were considered those that achieved an antibody response of 15 AU/mL or greater, therefore a second dose of vaccine increased the number of responders from 21% to 79% (compared to 53% to 100% in the general population). In a study conducted in Israel,36 11 myeloma patients that had undergone stem cell transplant and were considered immunocompromised were tested for antibody response 2-4 weeks after vaccination with the Pfizer/BioNTech vaccine. 74.8% of patients developed an antibody response to the vaccination. Furthermore, according to the authors, participants in the study did not experience any episodes of rejection, graft-versus-host disease (GVHD) or allergy.”36 In a study conducted in Germany,37 82 patients were tested for antibody response after their first dose of vaccination and only 23% were found to have a response. This research supports the need for completion of vaccination in myeloma patients along with the need for booster vaccinations.