What is CAR-T therapy?
CAR T-cell therapy is a form of immunotherapy that uses the patient’s own white blood cells (specifically, T-cells) to fight myeloma. T-cells play an important role in the body’s immune response to infection or cancer. Cancer cells commonly evade or hide from our body’s immune system, making them difficult to find and destroy. In CAR T-cell therapy, some of a patient’s T-cells are extracted through a pheresis, a procedure in which blood is withdrawn, the T-cells separated and the remainders returned to the circulation. The T-cells are then equipped with a protein molecule called “chimeric antigen receptor” (CAR) and reinfused to the patient. The CAR protein, a sort of antenna that guides the T-cells to cancer cells, sits on the surface of the modified T-cells and can instruct them to seek out cancerous myeloma cells, recognise a protein on their surface, bind to them and destroy them. This CAR protein is primarily expressed on myeloma cells and therefore does not attack normal cells.
CAR T-cell therapy is a multistep process requiring time for manufacturing and is given to patients through an infusion. For more information on CAR T-cell therapy and how it works, please see our CAR T-cell therapy Q&A and watch our CAR T-cell therapy educational clip.
A summary of MPE’s available resources on CAR T-cell therapy is also available here.
Am I eligible for CAR T-cell therapy?
As eligibility to CAR T-cell therapy vastly differs, it is advisable that you consult your doctor about whether CAR-T is appropriate for your myeloma. Most CAR-T products are currently being tested in clinical trials and there are several products on the market, which are approved for myeloma in the European Union. However, these treatments are only currently available in a few European countries. Also, most CAR-T patients have a myeloma that is considered relapsed/refractory. Ide-cel (Abecma®) and Cilta-cel (Carvykti®) are indicated for patients who have received three prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor and an anti CD38 antibody, and whose disease has worsened since the last treatment. In addition, patients must have good organ function, e.g., well-functioning kidneys, liver and heart. For more information on eligibility criteria for CAR-T, please see our CAR-T eligibility Q&A.
What CAR-T products are currently authorised in the EU?
As of January 2023, two CAR T-cell products are currently authorised in the European Union: idecabtagene vicleucel (Abecma, or ide-cel) and ciltacabtagene autoleucel (Carvykti, or cilta-cel). Both ide-cel and cilta-cel target an antigen or protein on the surface of myeloma cells known as B-cell maturation antigen (BCMA). You can find the factsheets on these specific treatments here. In addition to these products, multiple CAR-T drugs that target BCMA and various other antigens are at present in clinical development. The CARAMBA trial, for example, is currently investigating CAR T-cells that target the myeloma antigen protein SLAMF7. Other CAR-T products are currently in development.
What are CAR-T’s short-term side effects and how can these be managed?
There are multiple, possible, short-term side effects associated with CAR T-cell therapy, including:
- cytokine release syndrome (CRS)
- immune effector cell- associated neurotoxicity (ICANS)
- macrophage activation syndrome (MAS)
- haematologic (blood) toxicities
- hypogammaglobulinaemia
- infections
- gastrointestinal symptoms
These side effects and their management are discussed in greater detail below. It is important to remember that each patient may respond differently to CAR-T, so it is impossible to precisely predict which side effects you will experience and for how long. Most patients won’t experience them all.
Cytokine release syndrome (CRS)
After patients are given CAR T-cells through an infusion, the manufactured T-cells multiply in the body and release cytokines. Cytokines are small proteins that serve as messengers activating the immune system to kill myeloma cells. A large amount of cytokines released by CAR T-cells causes a robust inflammatory response known as cytokine release syndrome (CRS).
CRS can cause many symptoms, ranging from mild flu-like symptoms to severe, life-threatening reactions. In mild/moderate cases, patients may experience fever, headaches, body aches, fatigue, shortness of breath, and gastrointestinal symptoms such as nausea and diarrhoea. In severe cases, patients may experience these symptoms in addition to dangerously low blood pressure, a fast heart rate, altered levels of consciousness and organ failure1.
CAR T-cell therapy is also associated with cardiac (heart) toxicities, which mostly occur within the context of CRS. CRS is the strongest predictor of cardiac complications, along with pre-existing cardiac disease and high tumour burden2,3. The management of cardiac toxicities will depend on which specific type you are experiencing and the context in which you are experiencing symptoms (e.g., if you also have CRS). Types of cardiovascular complications that can occur include hypotension (low blood pressure), an irregular heart rhythm (arrhythmia) and cardiogenic shock (a life-threatening emergency where your heart cannot pump all the blood your body needs). Cardiovascular events are estimated to occur in anywhere between 10-36% of patients seeking CAR-T for a variety of conditions (not limited to myeloma)4. Moreover, tachycardia (a fast heart rate, which is a physiological symptom occurring in the case of fever and low blood pressure) can be quite frequent. In the CARTITUDE-1 trial studying 97 patients, tachycardia was reported in 27% of participants5, while in the KarMMa study of 128 patients, tachycardia was observed in 19%6.
In clinical trials, CRS is divided into four grades based on severity (grade 1 = mild, grade 2 = moderate, grade 3 = severe and grade 4 = life-threatening), with grade 5 being death. The grading system enables clinicians and researchers to accurately report the prevalence and severity of side effects. The most used grading system to date is the Common Terminology Criteria for Adverse Events (CTCAE, Figure 1)7. Other criteria for grading CRS have been proposed as well.
*These symptoms may also be experienced at higher grades in addition to the specific criteria listed for each grade above.
Figure 1. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 grade explanations for cytokine release syndrome7.
CRS is one of the most common side effects of CAR T-cell therapy. A meta analysis (a research study that looks at the results of several studies together) reviewed 23 BCMA CAR T-cell products administered to 640 myeloma patients. Among the 640 patients, 80% experienced some degree of CRS, with 14.3% experiencing CRS considered severe (grade 3 or higher)8. In the CARTITUDE-1 specifically evaluating the safety and efficacy of cilta-cel (Carvykti®) among 97 participants, CRS occurred in 94.8% of patients (mostly grade 1 or 2)9, with 5% of patients experiencing grade 3 or higher CRS. In the KarMMa-1 study evaluating ide-cel (Abecma®), among the 127 patients, CRS was seen in 85% (mostly grade 1 or 2)10, with 9% of patients experiencing grade 3 or higher CRS.
How soon CRS begins and how long it lasts can vary depending on the patient. However, CRS typically begins 1-14 days (often 2-3 days) after CAR-T infusion1. The duration can also vary depending on many factors, including the which CAR-T product is used and how their CRS is managed. Usually, however, CRS can be resolved in as little as a few days or up to 2-3 weeks1. In the CARTITUDE-1 trial, for example, CRS was resolved within 14 days of onset for 99% of patients, while in the subsequent CARTITUDE-2 trial, CRS was resolved within seven days for 90% of patients11.
Although CRS is a common side effect of CAR-T, in general it can be well-controlled using medications. CRS is most effectively managed with a combination of a steroid and tocilizumab (RoActemra®)1. Tocilizumab is a monoclonal antibody that regulates your immune response by blocking a specific protein called interleukin-6. For more information on tocilizumab, please see the European Medicines Agency (EMA) website here.
Immune effector cell-associated neurotoxicity syndrome (ICANS)
Immune effector cell-associated neurotoxicity syndrome (ICANS) is a potential neurological side effect of CAR T-cell therapy. Researchers hypothesise that ICANS occurs because of the high levels of immune system activity following CAR T-cell infusion. Inside our bodies, we have a protective layer between our bloodstream and brain called the blood-brain barrier (BBB). The BBB helps prevent harmful substances from reaching our brains, allowing necessary substances such as water and oxygen to pass through. The over-activation of the immune system after CAR-T infusions can disrupt the BBB, allowing harmful cytokines (a type of inflammatory chemical in the body) to enter the central nervous system (brain and spinal cord) with subsequent harmful effects12.
ICANS can cause various symptoms, including altered levels of consciousness, confusion, behaviour changes, hallucinations, difficulty speaking, difficulty with fine motor skills (e.g., writing), seizures and cerebral oedema (swelling of the brain) that may cause coma12.
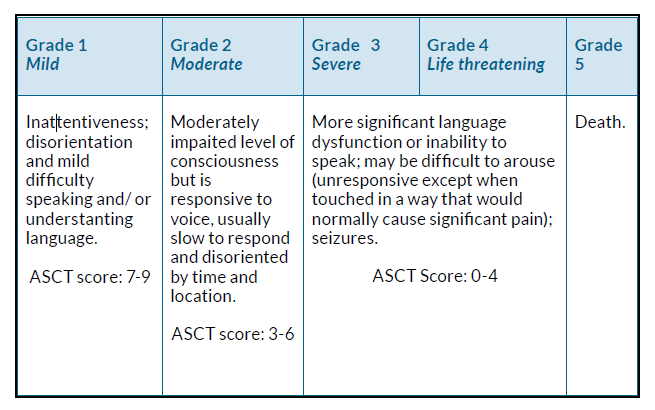
Like CRS, ICANS is divided into five grades based on severity (grade 1 = mild, grade 2 = moderate, grade 3 = severe and grade 4 = life-threatening), with grade 5 being death. Often ICANS is graded using either the American Society for Transplantation and Cellular Therapy (ASTCT) grading scale (a 10-point scoring system evaluating encephalopathy or level of confusion and associated symptoms) or the Common Terminology Criteria for Adverse Events (CTCAE)5.
Figure 2 provides a combined summary of the ASCT score and CTCAE.
Figure 2. Common Terminology Criteria for Adverse Events (CTCAE) and American Society for Transplantation and Cellular Therapy (ASTCT) grade explanations for immune effector cell-associated neurotoxicity syndrome (ICANS)12.
The onset of ICANS can depend on which CAR-T product you receive and varies by patient. Typically, ICANS occurs within 3-10 days of CAR T-cell infusion, and acute symptoms usually resolve within 7-10 days of onset. In most cases, ICANS occurs in patients also experiencing CRS as a side effect, however, this is not always the case12. Although ICANS occurs less frequently than CRS, it is still a common side effect. In the CARTITUDE-1 trial of 97 patients, 17% experienced ICANS, with 2% experiencing grade 3 or higher13. Similarly, in the KarMMa trial of 128 patients, 18% experienced ICANS, with 3% grade 3 or higher13. You may be more at risk of developing ICANS if you have a pre-existing neurological or medical condition, high disease burden, severe CRS that occurred soon after CAR T-cell therapy (early onset CRS), or if you are younger. ICANS is treated mainly with supportive care. You may be given a steroid or antiseizure medication. Depending on the severity of symptoms, you may be required to stay in the intensive care unit of the hospital while you recover12,14. Alternative medicines that may be used to treat ICANS are siltuximab (a monoclonal antibody that helps to regulate immune responses) and anakinra (an immunosuppressive medication that reduces the activity of the immune system).
Macrophage activation syndrome (MAS)
Macrophage activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis (HLH) results from the over-activation and dysregulation of the immune system. Macrophages are a specialised type of immune cell that help eliminate harmful microorganisms and diseased or damaged cells in our bodies. Macrophages do this by engulfing or ingesting harmful microorganisms to stimulate an immune response. Usually, macrophages in our bodies are regulated by other immune cells known as natural killer cells (NK cells) and cytotoxic lymphocytes. In MAS, however, the regulation process does not occur. Instead, macrophages are activated and release large amounts of cytokines, or inflammatory chemicals, which cause extensive tissue damage and potential organ failure. In addition, macrophages in MAS also ingest other important blood cells and components our bodies need15.
Your doctors may suspect you have MAS if you have a persistent fever even after your CRS symptoms have subsided. Other symptoms of MAS include:
- pancytopenia (low levels of several types of blood cells such as platelets, red blood cells, etc.)
- high levels of ferritin (a protein that stores iron)
- blood clotting issues
- liver dysfunction16
Although MAS appears to occur less frequently than other side effects of CAR-T, the condition can be life-threatening. In the CARTITUDE-1 trial, MAS occurred in 1% of patients (1 patient) who developed MAS 99 days after CAR T-cell therapy5. In the KarMMa-1 study evaluating ide-cel, MAS occurred in 4% of patients within ten days after CAR T-cell therapy. Two patients experienced fatal outcomes6. The most appropriate treatment for MAS has not yet been firmly established.
Currently, it is often treated with steroids or anakinra (an immunosuppressive medication that reduces the activity of the immune system). Haematologic (blood) toxicities CAR T-cell therapy is associated with several blood-related side effects, including cytopenia (a reduction in various types of blood cells). While CAR-T itself can cause cytopenia, patients often experience a decrease in blood cell counts due to the administration of chemotherapy before CAR T-cell therapy, which is an expected side effect and can be managed.
In our bodies, there are three main types of blood cells:
- red blood cells (erythrocytes, which carry oxygen and nutrients)
- white blood cells (leukocytes that fight infection)
- platelets, which are essential for blood clotting
The levels of any or all of these blood cells can be reduced during CAR T-cell therapy. The symptoms you may experience will depend on which of your blood cells are affected.
Patients who experience low levels of red blood cells (anaemia) may experience any of the following:
- fatigue
- weakness
- light headedness
- poor concentration
- shortness of breath
Patients with low white blood cells (leukopenia) may, consequently, experience frequent infections with fever and other complaints depending on the site of the infection as a consequence. Patients with low levels of platelets (thrombocytopenia) may bruise or bleed easily as platelets help blood clot.
Blood-related side effects are very common after CAR T-cell therapy. In the CARTITUDE-1 study evaluating the safety and efficacy of cilta-cel (Carvykti), among the 97 participants who received the treatment, 96% developed neutropenia, 81% anaemia and 79% percent thrombocytopenia9. In the KarMMa-1 study evaluating ide-cel, among 128 patients, cytopenia was the most common side effect reported in 97% of patients10. In the KarMMA-2 trial, looking at the efficacy and safety of ide-cel in 31 clinical high-risk myeloma
patients, 80.6% developed neutropenia, 29% leukopenia and 22.6% anaemia17.
Because blood toxicities are common after CAR T-cell therapy, your blood counts will be monitored before, during and frequently after treatment. Management of cytopenia may require administering medications called growth factors that stimulate the development of blood cells in the bone marrow. In severe cases of cytopenia, some patients may require blood product transfusions.
Often hematologic side effects are seen initially and throughout treatment with CAR-T. It is important to know, however, that for some patients, changes in blood and cell counts may be prolonged or recur after they are resolved. For more information, please see the question on long-term CAR-T side effects below.
Hypogammaglobulinaemia
CAR T-cell therapy may cause you to develop hypogammaglobulinaemia (low levels of immunoglobulins [IgG] and antibodies in your blood). Immunoglobulins and antibodies are proteins that help defend our bodies against bacterial and viral infections (including upper respiratory tract infections [URIs]). While CAR T-cells are designed to attack myeloma cells, they may target healthy plasma cells that produce normal antibodies as well, resulting in hypogammaglobulinaemia14.
Hypogammaglobulinaemia is often seen after treatment with CAR T-cell therapy. In a study of 127 patients, 27 (21%) experienced hypogammaglobulinaemia as a side effect of treatment with ide-cel6. Similarly, in a study of cilta-cel, 12% (12/97) experienced hypogammaglobulinaemia5.
Because of the risks of severe infections associated with hypogammaglobulinaemia, your IgG levels will be monitored before and after CAR T-cell therapy. Hypogammaglobulinaemia may be present while relevant levels of CAR T-cells are found in peripheral blood.
The management of hypogammaglobulinaemia varies, however, it may include receiving intravenous immunoglobulins (IVIG infusions) containing antibodies. In addition, management may include taking precautions to reduce the risk of infections and possibly prophylactic or preventative treatments such as antibiotics or antivirals.
Infections
Patients who receive CAR T-cell therapy are often at an increased risk of infection due to multiple factors, including treatments that suppress the immune system, low white blood cell levels and hypogammaglobulinaemia (see above). Often, infections that are rarely seen in healthy individuals may occur after CAR-T as patients are considered immunocompromised. Therefore, these infections may be a bit harder to treat.
Before CAR-T, you will receive conditioning therapy (also called lymphodepletion chemotherapy, a treatment to reduce the number of circulating lymphocytes, prior to infusion of CAR T-cells) to help your body accept the CAR T-cells. Conditioning treatments are necessary for CAR-T to be effective. However, they may make you more susceptible to infection, due to low blood cells and immune cells. In addition, medications such as tocilizumab or steroids used to manage cytokine release syndrome (CRS) and other CAR-T side effects can suppress your immune system.
If you develop an infection during or after CAR T-cell therapy, you may experience more severe outcomes because of your weakened immune system. COVID-19, for example, can be particularly severe in patients who have received CAR-T. Research has shown myeloma patients who contracted COVID-19 after CAR T-cell therapy may experience a poor outcome (with a 15 times higher risk of death) and may require longer isolation times2. This probably also accounts for other respiratory viral infections.
Infections are most common in the first 30 days following CAR T-cell infusion2. During this time, you may be advised to take precautions such as frequent handwashing, wearing a mask, social distancing and avoiding sick contacts to limit your risk of infection. If you develop an infection, the treatment will depend on which specific type of infection it is – bacterial or viral. Given that infections are common following CAR T cell infusion, some doctors will recommend prophylactic (or preventative) treatment using antibiotics or antivirals.
Gastrointestinal symptoms
Like many treatments, CAR T-cell therapy can cause significant gastrointestinal (GI) symptoms such as a loss of appetite, diarrhoea, constipation, nausea and vomiting. In the CARTITUDE-1 trial, studying 97 patients, GI symptoms occurred in approximately one out of three patients, with diarrhoea and nausea being the most common (diarrhoea 33%, nausea 31%, constipation 22% and vomiting 20%)5. Similar frequencies were seen in the KarMMa trial, where 35% of patients developed diarrhoea, 29% nausea, 16% constipation, 15% vomiting and 12% oral pain6.
The management of GI symptoms will often vary but may include oral and intravenous medicines to slow the rate of diarrhoea and ease nausea. It is often important to note that GI symptoms can also cause electrolyte disturbances such as hyponatraemia or hypokalaemia (low sodium or potassium) in your blood, which can be dangerous if left untreated.
Other side effects
The list of side effects included above is not exhaustive, and patient reports highlight the diversity of side effects they may experience. Parkinsonism, for example, is a potential side effect of CAR T-cell therapy that may be concerning for patients. Symptoms of parkinsonism may include slow, involuntary or repetitive movements, muscle weakness, difficulty speaking and/or swallowing, changes in reflexes and other motor symptoms.
Symptoms appeared between 15 and 108 days after CAR T-cell infusion. Three patients in the CARTITUDE-1 trial investigating cilta-cel experienced grade ≥3 parkinsonism18. Moreover, preliminary results from the CARTITUDE-4 trial indicate that the treatment is better tolerated when used earlier in the course of the disease.
Because of the many different types of side effects that can occur after CAR T-cell therapy, it is important to report any changes in how you feel to your doctor, so that they can best manage any side effects that occur.
What are the long-term side effects of CAR-T? How can these be managed?
Although many of the side effects of CAR-T occur soon after treatment, other side effects may last longer. Since every patient may respond differently, it is impossible to predict which side effects you will experience.
However, possible long-term side effects include:
- Prolonged haematologic (blood) changes: Following CAR-T, patients may experience low levels of blood cells for significant periods. In a study of 54 myeloma patients who underwent CAR T-cell therapy with anti-BCMA and an anti-CD-19 CAR T- cell product, 52% experienced prolonged changes in their blood levels 28 days after treatment:
- 46% severe neutropenia (low neutrophils, a type of white blood cell)
- 30% severe anaemia (low red blood cells)
- 31% severe thrombocytopenia (low platelets for clotting).
This same study showed that patients with prolonged blood changes also had a poorer prognosis19.
- Weakened immune system and higher risk of infections: Prolonged low levels of white blood cells and immunoglobulins/antibodies (hypogammaglobulinaemia), which may make you more susceptible to various severe infections and at risk for worse outcomes
- Emergence of new or worsening of existing auto-immune toxicities (very rare)
- Development of recurrent or second primary cancers
What other indirect side effects associated with CAR-T should I be aware of?
CAR T-cell therapy can significantly impact patients’ daily lives physically, mentally, financially and psychologically. For example, many patients report long-term fatigue and difficulty returning to work after CAR-T. A global patient survey conducted by the Lymphoma Coalition found that 81% of surveyed lymphoma patients experienced fatigue after CAR-T and only 57% of doctors followed up on this symptom2. Research shows that patients may be reluctant to discuss fatigue and psychological symptoms such as depression, fear, anxiety, decreased libido, or sex drive with their doctors2. It is important to have these
conversations with your providers, so that they can help you and make sure your needs are addressed.
CAR T-cell therapy may also significantly impact your relationship with your carers and their daily life. Carers will be very involved in your care, particularly in the first month after CAR-T, as you must have someone with you 24 hours a day. Furthermore, you and your carer may have to miss work and travel long distances to treatment centres or stay away from home for treatment with CAR-T, if a centre is not immediately available in your area. This may also have a financial impact and plans must be made before starting treatment with CAR-T.
Are there any general safety measures implemented by the regulatory bodies to minimise the risk of patients experiencing side effects?
As time goes on, the administration of CAR-T and the management of side effects such as cytokine release syndrome (CRS) are becoming more and more harmonised. The European Group for Blood and Marrow Transplantation (EBMT) recommends that CAR-T is delivered at accredited centres for haematopoietic cell transplantation, and the EBMT registry is now registering and monitoring European CAR-T recipients. Together with the European Haematology Association (EHA), the EBMT has also established the GoCART consortium to harmonise standards, guidelines and regulatory requirements across the European Union with plans to publish recommendations20. In 2022, EBMT and EHA have published best practice recommendations based on the current literature to support health care professionals in delivering consistent and high-quality care to patients receiving CAR T-cell therapy3. These include recommendations on eligibility assessment, screening tests, leukapheresis, bridging therapy (administered between leukapheresis and CAR-T infusion), hospitalisation, lymphodepletion, handling and storage of the product, side effect management and long-term follow-up.
In addition to the above, CAR-T is considered a gene therapy medicinal product or advanced medicinal product (ATMP) due to the use of the chimeric antigen receptor (CAR) gene insertion into human T-cells. Therefore, the EMA often centrally authorises and continues to monitor the safety and efficacy of CAR-T products, and provides researchers with scientific support to help design risk management systems and monitor side effects and CAR-T safety21. The EBMT is also conducting post-authorisation studies (PAS) that will allow to better understand the efficacy, toxicity and side effects of most of the approved autologous CAR T-cell treatments in real life.
What precautions do I have to take before receiving CAR-T?
As outlined above, before receiving CAR T-cell therapy, you will be given conditioning therapy (also called lymphodepletion chemotherapy) that helps your body accept the CAR-T. Conditioning treatments are necessary for CAR-T to be effective. However, conditioning treatments may make you more susceptible to infection, and you may be advised to follow extra precautions to prevent infections, such as handwashing, wearing a mask and limiting exposure to sick contacts while awaiting your CAR T-cell therapy. During the time that you receive conditioning treatment, you may also feel worse, and there is a risk that your myeloma may worsen to the point that your general health deteriorates so much that you are no longer eligible for CAR-T. CAR T-cell therapy requires an extended hospital stay, which you should prepare for. You will also want to be sure that you and your carers are educated and prepared for any possible side effects of treatment. Likewise, you must have adequate physical care and psychological support upon your return home.
Finally, before entering the hospital, you may want to make necessary arrangements for the possibility that CAR T-cell therapy does not work for you as planned. Or, that you may experience severe side effects, making you unable to make medical decisions for yourself. Therefore, it is essential to talk to your carers about your wishes and potentially complete an advanced directive detailing your desire for medical treatment in these situations.
What precautions do I have to take after receiving CAR-T?
After your CAR T-cell therapy, you will have a weakened immune system, and you may be advised to continue taking precautions to limit your risk of infection, including COVID-19 exposure. You may be required to monitor your temperature frequently for signs of infection.
Low blood cell counts are expected (as described above) and may put you at risk of fatigue, bruising and bleeding easily. You may want to take precautions, for example, when using sharp tools, including shaving razors, to avoid cutting yourself and removing common tripping hazards to prevent falls.
For the first month after CAR T-cell therapy, it is crucial to have someone with you 24 hours a day. Your carer will be able to monitor you for side effects and help administer medications appropriately. In addition, you will ideally need to stay within 30 minutes to one hour driving distance of the treatment location (if possible) and have a carer who can drive you wherever you need to go for two months after treatment.
What can my caregiver(s), friends and family do to support me before, during and after I undergo CAR T-cell therapy?
Support from caregivers is vital during the CAR T-cell therapy process. Support might be needed before treatment, when information about the treatment, potential side effects and how to prepare for the therapy is given to you. After treatment, you will require frequent and close monitoring. Therefore, carer involvement is critical as they can administer medications, contact your treating clinician and monitor you for any warning signs such as confusion or fever. For further information on how your carers, friends and family can support you throughout your CAR T-cell therapy, please see our Q&A on CAR T-cell therapy.
References:
- Cytokine release syndrome (CRS) – UpToDate. Accessed December 31.
- Myeloma Patients Europe, Conference reports, 4th-European-CART- cell-Meeting. Myeloma Patients Europe. Accessed March 10, 2023.
- Hayden PJ, Roddie C, Bader P, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol Off J Eur Soc Med Oncol. 2022;33(3):259-275. doi:10.1016/j.annonc.2021.12.003
- Patel NP, Doukas PG, Gordon LI, Akhter N. Cardiovascular Toxicities of CAR T-cell Therapy. Curr Oncol Rep. 2021;23(7):78. doi:10.1007/s11912-021-01068-0
- Research C for BE and. CARVYKTI. FDA. Published online March 21, 2022. Accessed January 31, 2023.
- Research C for BE and. ABECMA (idecabtagene vicleucel). FDA. Published online April 21, 2021. Accessed January 31, 2023.
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j. bbmt.2018.12.758
- Roex G, Timmers M, Wouters K, et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J Hematol OncolJ Hematol Oncol. 2020;13(1):164. doi:10.1186/s13045-020-01001-1
- Thomas Martin et al., ‘Updated Results from CARTITUDE-1: Phase 1b/2Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen- Directed Chimeric Antigen Receptor T Cell Therapy, in Patients With Relapsed/Refractory Multiple Myeloma’, Blood 138, no. Supplement 1 (5 November 2021): 549–549,
- Larry D. Anderson J, Munshi NC, Shah N, et al. Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: Updated KarMMa results. J Clin Oncol. Published online May 28, 2021. doi:10.1200/JCO.2021.39.15_suppl.8016
- CARVYKTI – Adverse Event – Cytokine Release Syndrome (CRS). Accessed March 25, 2023.
- Immune effector cell-associated neurotoxicity syndrome (ICANS) – UpToDate. Accessed January 2, 2023.
- Rates of ICANS in pivotal CAR-T trials – UpToDate. Accessed January 14, 2023.
- Rendo MJ, Joseph JJ, Phan LM, DeStefano CB. CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Challenges. Blood Lymphat Cancer Targets Ther. 2022;12:119-136. doi:10.2147/BLCTT.S327016
- Clinical features and diagnosis of hemophagocytic lymphohistiocytosis– UpToDate. Accessed January 2, 2023.
- Crayne CB, Albeituni S, Nichols KE, Cron RQ. The Immunology of Macrophage Activation Syndrome. Front Immunol. 2019;10:119. doi:10.3389/fimmu.2019.00119
- Dhodapkar M, Alsina M, Berdeja J, et al. KarMMa-2 Cohort 2c: Efficacy and Safety of Idecabtagene Vicleucel in Patients with Clinical High-Risk Multiple Myeloma Due to Inadequate Response to Frontline Autologous Stem Cell Transplantation. Blood. 2022;140(Supplement 1):7441-7443. doi:10.1182/blood-2022-162615
- Van Oekelen O, Aleman A, Upadhyaya B, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism following BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27(12):2099-2103. doi:10.1038/s41591-021-01564-7
- Li H, Zhao L, Sun Z, et al. Prolonged hematological toxicity in patients receiving BCMA/CD19 CAR-T-cell therapy for relapsed or refractory multiple myeloma. Front Immunol. 2022;13:1019548. doi:10.3389/
fimmu.2022.1019548 - GoCART Coalition. EBMT. Published January 24, 2023. Accessed February 9, 2023.
- EMA. Advanced therapy medicinal products: Overview. European Medicines Agency. Published September 17, 2018. Accessed February 9, 2023.